PSAT Math Multiple-Choice Question 715: Answer and Explanation
Question: 715
The ideal gas equation is PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature. According to the equation, the volume of a gas is inversely related to
- A. the number of moles.
- B. gas constant.
- C. temperature.
- D. none of the above.
Correct Answer: D
Explanation:
(D) Solve for V:

Recall that, in general, y is directly proportional to x if y = cx for some constant c, and y is inversely proportional if 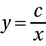 . In our case, we are told that R is the gas constant, so we can think of this as our constant. So V is directly proportional to n> and T. If you increased either of these variables, V would also increase. V is inversely proportional only to P, which isn't an answer choice. So choice (D) is the correct answer.
. In our case, we are told that R is the gas constant, so we can think of this as our constant. So V is directly proportional to n> and T. If you increased either of these variables, V would also increase. V is inversely proportional only to P, which isn't an answer choice. So choice (D) is the correct answer.
Test Information
- Use your browser's back button to return to your test results.
- Do more Math Multiple-Choice Tests tests.
More Tests
- PSAT Math Multiple-Choice Test 1
- PSAT Math Multiple-Choice Test 2
- PSAT Math Multiple-Choice Test 3
- PSAT Math Multiple-Choice Test 4
- PSAT Math Multiple-Choice Test 5
- PSAT Math Multiple-Choice Test 6
- PSAT Math Multiple-Choice Test 7
- PSAT Math Multiple-Choice Test 8
- PSAT Math Multiple-Choice Test 9
- PSAT Math Multiple-Choice Test 10
- PSAT Math Multiple-Choice Test 11
- PSAT Math Multiple-Choice Test 12
- PSAT Math Multiple-Choice Test 13
- PSAT Math Multiple-Choice Test 14
- PSAT Math Multiple-Choice Test 15
- PSAT Math Multiple-Choice Test 16
- PSAT Math Multiple-Choice Test 17
- PSAT Math Multiple-Choice Test 18
- PSAT Math Multiple-Choice Test 19
- PSAT Math Multiple-Choice Test 20
- PSAT Math Multiple-Choice Test 21
- PSAT Math Multiple-Choice Test 22
- PSAT Math Multiple-Choice Test 23
- PSAT Math Multiple-Choice Test 24
- PSAT Math Multiple-Choice Test 25