PSAT Math Multiple-Choice Question 669: Answer and Explanation
Question: 669
The root mean squared speed of a molecule, vrms, is calculated using the formula 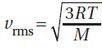 , where R is a gas constant, T is the temperature, and M is the molecular mass. The molecular mass of substance A is most likely to be less than the molecular mass of substance B if the temperature and vrms of substance A compare in which ways to those of substance B ?
, where R is a gas constant, T is the temperature, and M is the molecular mass. The molecular mass of substance A is most likely to be less than the molecular mass of substance B if the temperature and vrms of substance A compare in which ways to those of substance B ?
- A. Greater vrms and lower temperature
- B. Lower vrms and greater temperature
- C. Lower vrms and equal temperature
- D. Cannot be determined
Correct Answer: A
Explanation:
(A) The relationship is easiest to see if you solve for M first. First, square both sides:
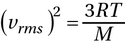
Multiply both sides by M to get M out of the denominator:
M(vrms)2 = 3RT
Now isolate M:

We can now consider how we can lower M. First, M is directly proportional to T. So decreasing T will decrease M. Further, M is inversely proportional to the square of vrms. So increasing vrms will decrease M because you will be dividing by a larger number. Therefore, choice (A) is correct.
Test Information
- Use your browser's back button to return to your test results.
- Do more Math Multiple-Choice Tests tests.
More Tests
- PSAT Math Multiple-Choice Test 1
- PSAT Math Multiple-Choice Test 2
- PSAT Math Multiple-Choice Test 3
- PSAT Math Multiple-Choice Test 4
- PSAT Math Multiple-Choice Test 5
- PSAT Math Multiple-Choice Test 6
- PSAT Math Multiple-Choice Test 7
- PSAT Math Multiple-Choice Test 8
- PSAT Math Multiple-Choice Test 9
- PSAT Math Multiple-Choice Test 10
- PSAT Math Multiple-Choice Test 11
- PSAT Math Multiple-Choice Test 12
- PSAT Math Multiple-Choice Test 13
- PSAT Math Multiple-Choice Test 14
- PSAT Math Multiple-Choice Test 15
- PSAT Math Multiple-Choice Test 16
- PSAT Math Multiple-Choice Test 17
- PSAT Math Multiple-Choice Test 18
- PSAT Math Multiple-Choice Test 19
- PSAT Math Multiple-Choice Test 20
- PSAT Math Multiple-Choice Test 21
- PSAT Math Multiple-Choice Test 22
- PSAT Math Multiple-Choice Test 23
- PSAT Math Multiple-Choice Test 24
- PSAT Math Multiple-Choice Test 25