PSAT Math Multiple-Choice Question 212: Answer and Explanation
Question: 212
In chemistry, the combined gas law formula 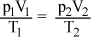 gives the relationship between the volumes, temperatures, and pressures for two fixed amounts of gas. Which of the following gives p2 in terms of the other variables?
gives the relationship between the volumes, temperatures, and pressures for two fixed amounts of gas. Which of the following gives p2 in terms of the other variables?
- A.
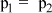
- B.
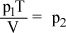
- C.
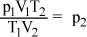
- D.
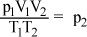
Correct Answer: C
Explanation:
C
Difficulty: Easy
Category: Passport to Advanced Math/Rational Equations
Getting to the Answer: Focus on the question at the very end—it's just asking you to solve the equation for p2. Multiply both sides by T2 to get rid of the denominator on the right-hand side of the equation. Then divide by V2 to isolate p2:
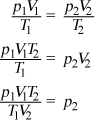
Stop here! You cannot cancel the V s and T s because the subscripts indicate that they are not the same variable. In math, subscripts do not behave the same way superscripts (exponents) do. Choice (C) is correct.
Test Information
- Use your browser's back button to return to your test results.
- Do more Math Multiple-Choice Tests tests.
More Tests
- PSAT Math Multiple-Choice Test 1
- PSAT Math Multiple-Choice Test 2
- PSAT Math Multiple-Choice Test 3
- PSAT Math Multiple-Choice Test 4
- PSAT Math Multiple-Choice Test 5
- PSAT Math Multiple-Choice Test 6
- PSAT Math Multiple-Choice Test 7
- PSAT Math Multiple-Choice Test 8
- PSAT Math Multiple-Choice Test 9
- PSAT Math Multiple-Choice Test 10
- PSAT Math Multiple-Choice Test 11
- PSAT Math Multiple-Choice Test 12
- PSAT Math Multiple-Choice Test 13
- PSAT Math Multiple-Choice Test 14
- PSAT Math Multiple-Choice Test 15
- PSAT Math Multiple-Choice Test 16
- PSAT Math Multiple-Choice Test 17
- PSAT Math Multiple-Choice Test 18
- PSAT Math Multiple-Choice Test 19
- PSAT Math Multiple-Choice Test 20
- PSAT Math Multiple-Choice Test 21
- PSAT Math Multiple-Choice Test 22
- PSAT Math Multiple-Choice Test 23
- PSAT Math Multiple-Choice Test 24
- PSAT Math Multiple-Choice Test 25